
JEXPO and VOCLET Chemistry Model Set 1
JEXPO and VOCLET Chemistry Model Set 1
‘Chemistry’ is the branch of science involved with compounds composed of atoms, i.e. elements, and molecules, i.e. combinations of atoms: their composition, structure, properties, behavior and the changes they undergo during a reaction with other compounds. It is one of the fundamental branches of science that arrangements with the properties, structure, and constituents of issue or substances and its communications with different types of issue. There is a great deal of contrast that can be seen when you think about Basic Chemistry and Modern science. Fundamental Chemistry began in antiquated circumstances because of popular researchers called Alchemists. It clarified every one of the essentials of the subject which were closed after a progression of examinations which shed light on how they truly functioned. Though Modern Chemistry won in the seventeenth century which laid orderly strides in picking up information about the issue. Present day science realized different hypotheses which at last ended up defended with the investigations they led to demonstrate how matter cooperates.
The space of Chemistry involves the investigation of the piece of the issue, its structure, and properties. The principal constituents of issue, iotas, and particles, shape the premise of science. Science has given responses to different inquiries. The quantitative estimation of the constituents of essential constituents of issue i.e. iotas and atoms and their reliance on the mass of the given issue is all around clarified by science. Advances in science have helped researchers and designers to bridle the vitality related with these iotas and atoms.
Today we can protect nourishment things for long term’s of time; we have a huge number of chemicals that are being utilized as a part of various enterprises for assembling an assortment of items. Phone covers, plastic sacks, the steel utilized as a part of development, the sanitized drain, photosynthesis, the saltines we consume, and so forth are just a couple of cases of results of science. These items are such profoundly established in our lives that we neglect to envision their significance and the part of the science behind them.
Q.1. Acetyl salicylic acid is commonly used as
a) A sedative
b) A fertilizer
c) A painkiller
d) Tear gas
Answer
Correct Answer: (c)
Q.2. Gasoline is the name given to the same substance as
a) Crude oil
b) Natural gas
c) Diesel oil
d) Petrol
Answer
Correct Answer: (d)
Q.3. Boric acid is a/an
a) Antibiotic
b) Germicide
c) Mild antiseptic
d) Strong antiseptic
Answer
Correct Answer: (c)
Q.4. Cholesterol is a
a) Derivative of chloroform
b) Type of chlorophyll
c) Fatty alcohol found in animal fats
d) Chromium salt
Answer
Correct Answer: (c)
Q.5. What is the volume of 1.6 gm of methane gas at N.T.P? Calculate the number of hydrogen atoms in it.
a) 22.4 litres, 2.4 × 1022
b) 22.4 litres, 2.4 × 1023
c) 2.24 litres, 2.4 × 1022
d) 2.24 litres, 2.4 × 1023
Answer
Correct Answer: (d)
Soln
Part – 1
Molecular weight of Methane (CH4) = 12 + 1 × 4 = 16 gms.
∴ At N.T.P. volume of 16 gms methane = 22.4 litres
∴ At N.T.P. volume of 1.6 gms of methane
=22.4×1.616=2.24 litres (Ans.)Part – 2
16 gms of methane contains 6.023 × 1023 number of methane molecules
∴ 1.6 gms of methane contains
=6.023×1023×1.616=6.023×1022 number of methane molecules∵ In 1 molecule of methane gas (CH4) contains = 4 hydrogen atoms.
∴ 6.023 × 1022 molecules of methane contains = 4 × 6.023 × 1022 = 2.4092 × 1023 number of hydrogen atoms. (Ans.)
Q.6. Sweet contains
a) Phosphoric acid
b) Pure water
c) Water, salt and waste matter
d) Calcium phosphate and water
Answer
Correct Answer: (c)
Q.7. Riboflavin is a/an
a) Antibiotics
b) Plant
c) Vitamin
d) Colouring substance
Answer
Correct Answer: (c)
Q.8. The pair of bases in natural nucleic acids which is held by hydrogen bonds is
a) Uracil and thymine
b) Adenine and thymine
c) Guanine and cytosine
d) Guanine and thymine
Answer
Correct Answer: (b)
Q.9. The substance that contains the maximum amount of nitrogen is
a) Ammonium chloride
b) Urea
c) Ammonium Nitrate
d) Ammonium Sulphate
Answer
Correct Answer: (b)
Q.10. Water pollution is caused by
a) Molasses
b) Sodium chloride
c) Industrial Wastes
d) Calcium carbonate
Answer
Correct Answer: (c)
Q.11. Indigo is a
a) Basic dye
b) Ingrain dye
c) Basic detox
d) Vat dye
Answer
Correct Answer: (d)
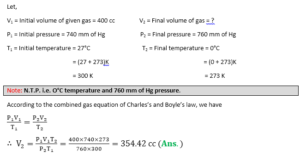
Q.12. The volume of a given mass of a gas is 400 CC at 27°C and 740 mm of Hg. pressure. What is its volume at N.T.P.?
a) 354.42 cc
b) 345.42 cc
c) 3454.2 cc
d) 3.5442 cc
Answer
Correct Answer: (a)
Soln
Q.13. The company ‘RANBAXY’ manufacturers
a) Heavy chemicals
b) Textiles
c) Plastics
d) Drugs
Answer
Correct Answer: (d)
Q.14. The drug against infectious diseases is
a) Aspirin
b) Reserpine
c) Insulin
d) Sulfathiazole
Answer
Correct Answer: (d)
Q.15. The drug is known as ‘vasodilators’ are used to treat
a) Hypertension
b) Cancer
c) Ulcers
d) AIDS
Answer
Correct Answer: (a)
Q.16. An example of a ‘cross-linked polymer’ is
a) PVC
b) Nylon
c) Polythene
d) Bakelite
Answer
Correct Answer: (d)
Q.17. The compound used as antifreeze is
a) Water
b) Methanol
c) Ethyl alcohol
d) Glycol
Answer
Correct Answer: (d)
Q.18. Interferon do not inhibit
a) Fungi
b) Viruses
c) Microbes
d) Bacteria
Answer
Correct Answer: (b)
Q.19. A mixture of glucose and fructose mixed in equal proportions is called
a) Brown sugar
b) Cane sugar
c) Invert sugar
d) Sucrose
Answer
Correct Answer: (c)
Q.20. What are the number of atoms in 16 gm of oxygen?
a) 6.032 × 1032
b) 6.023 × 1032
c) 6.032 × 1023
d) 6.023 × 1023
Answer
Correct Answer: (d)
Molecular mass of Oxygen (O2) = 16 × 2 = 32 gms
According to Avogadro’s law,
Number of molecules in 32 gms of Oxygen (O2) = 6.023 × 1023
∴ Number of molecules in 16 gms of oxygen = (6.023 × 1023 x 16 gms)⁄32 gms = 6.023 × 1023⁄2
Since, Oxygen (O2) is a diatomic gas i.e. 1 molecule of oxygen contains 2 oxygen atoms.
∴ Number of oxygen atoms in 6.023 × 1023⁄2 molecules of oxygen are = (6.023 × 1023⁄2) × 2 = 6.023 × 1023 ………..(Ans.)
Q.21. Chemically, interferon is a
a) Glycoprotein
b) Nucleic acid
c) Carbohydrate
d) Fluorinated hydrocarbon
Answer
Correct Answer: (a)
Q.22. The decolorizer used in sugar refining is
a) Sulphur dioxide
b) Hydrogen peroxide
c) Boneblack
d) Chlorine water
Answer
Correct Answer: (c)
Q.23. Chlorine water and carbon tetrachloride will produce a violet lower layer on mixing the ion
a) Iodide
b) Chloride
c) Bromide
d) Fluoride
Answer
Correct Answer: (a)
Q.24. The chemical ‘styrene’ is industrially used in the production of
a) Plastics
b) Dyes
c) Insecticides
d) Pharmaceuticals
Answer
Correct Answer: (a)
Q.25. ‘Aqua regia’ is a mixture of
a) HCl and HF
b) HCl and HBr
c) HCl and H2SO4
d) HCl and HNO3
Answer
Correct Answer: (d)
Q.26. Cooking gas is a mixture of
a) Carbon dioxide
b) Butane and propane
c) Methane and Ethylene
d) Carbon monoxide
Answer
Correct Answer: (b)
Q.27. The gas inside an electric bulb is
a) Nitrogen
b) Oxygen
c) Carbon dioxide
d) Air
Answer
Correct Answer: (a)
Q.28. Cooking oil can be converted into vegetable ghee by the process of
a) Crystallization
b) Oxidation
c) Distillation
d) Hydrogenation
Answer
Correct Answer: (d)
Q.29. The element present in the largest amount of Rocks and Minerals is
a) Hydrogen
b) Gold
c) Carbon
d) Silicon
Answer
Correct Answer: (d)
Q.30. Soap is prepared by heating caustic soda with
a) Almond oil
b) Linseed oil
c) Petroleum
d) Kerosene
Answer
Correct Answer: (b)
Q.31. The substance that is added to make neutral rubber strong and more buoyancy is
a) Sulphur
b) Polythene
c) Sponge
d) Chlorine
Answer
Correct Answer: (a)
Q.32. The term pH denotes the
a) Vapour pressure of the solution
b) Acidity or basicity of the solution
c) The ionic strength of the solution
d) Temperature of solution
Answer
Correct Answer: (b)
Q.33. Find the volume of one mole atom of oxygen, if its volume is half the volume of a mole of water.
a) 22.4 litres
b) 11.2 litres
c) 23.24 litres
d) 24.3 litres
Answer
Correct Answer: (b)
We know,
Volume of 1 mole of water (vapour) at N.T.P. = 22.4 litres
Therefore, Volume of half mole of water at N.T.P. = 22.4/2 = 11.2 litres
As we know that, oxygen is a diatomic gas i.e. 1 mole of oxygen contains 2 mole of oxygen atoms
Again, we know that
Volume of 1 mole oxygen at N.T.P. = 22.4 litres
Therefore, 11.2 litres of oxygen contains = 22.4/11.2 = 1/2 mole oxygen gas = 1 mole oxygen atom
Therefore, Volume of 1 mole oxygen atom at N.T.P is 11.2 litres. (Ans.)
Q.34. ‘Gobar gas’ contains mainly
a) Ethylene
b) Methane
c) Carbon dioxide
d) Acetylene
Answer
Correct Answer: (b)
Q.35. For plant growth, the most important compounds are made up of
a) Nitrogen
b) Sulphur
c) Oxygen
d) Carbon
Answer
Correct Answer: (a)
Q.36. The Antidote for lead poisoning is
a) EDTA
b) Cisplatin
c) Nickel
d) White of egg
Answer
Correct Answer: (a)
Q.37. The raw material not found in nature is
a) Petrol
b) Water
c) Vinyl chloride
d) Carbon
Answer
Correct Answer: (c)
Q.38. For the production of iron the raw material used in
a) Petrol
b) Coke
c) Rubber
d) Limestone
Answer
Correct Answer: (b)
Q.39. The substance that is not polymeric in nature is
a) Cellulose
b) Starch
c) Nylon
d) Glucose
Answer
Correct Answer: (d)
Q.40. River water is harder than rain-water because of it
a) contains salts of calcium and magnesium
b) contains sodium chloride
c) is exposed to the atmosphere
d) is always flowing
Answer
Correct Answer: (a)
Q.41. 200 ml of a gas at S.T.P weight 0.576 gm and weight of 1 litre of hydrogen at S.T.P is 0.09 gm. Calculate the vapour density of the gas and hence obtain its molecular weight.
a) 34, 62
b) 32, 64
c) 36, 24
d) 43, 26
Answer
Correct Answer: (b)

Q.42. River water is harder than rain-water because it
a) Contains salts of calcium and magnesium
b) Contains sodium chloride
c) Is exposed to the atmosphere
d) Is always flowing
Answer
Correct Answer: (a)
Q.43. Serpasil is
a) A mordant dye
b) Produced by microorganisms
c) Tranquilizer
d) Not a natural product
Answer
Correct Answer: (c)
Q.44. The pure crystalline form of silica used in scientific apparatus for passing ultraviolet light is called
a) Pyrex glass
b) Corning glass
c) Soda glass
d) Quartz glass
Answer
Correct Answer: (d)
Q.45. The hottest part of the gas flame is known as the
a) Non-luminous zone
b) Dark zone
c) Blue zone
d) Luminous zone
Answer
Correct Answer: (a)
Q.46. The compound used as an anti-material drug is
a) Aspirin
b) Penicillin
c) Hydroquinone
d) Chloroquine
Answer
Correct Answer: (d)
Q.47. The gas used for Artificial ripening of green fruits is
a) Ethane
b) Ethylene
c) Acetylene
d) Carbon dioxide
Answer
Correct Answer: (c)
Q.48. The first metal to be used by man was
a) Copper
b) Iron
c) Silver
d) Aluminium
Answer
Correct Answer: (a)
Q.49. Radiocarbon dating is used to estimate the ages of
a) Rocks
b) Fossils
c) Ancient buildings
d) Babies
Answer
Correct Answer: (c)
Q.50. Of the following substances, the one that can be used as an explosive is
a) TNT
b) DDT
c) Ozone
d) Paracetamol
Answer
Correct Answer: (a)
JEXPO and VOCLET Chemistry Model Set 1
JEXPO and VOCLET Chemistry Model Set 2
JEXPO and VOCLET Chemistry Model Set 3
Subscribe my Youtube Channel : Science Duniya in Bangla
JEXPO and VOCLET Chemistry Model Set 1, JEXPO and VOCLET Chemistry Model Set 1, JEXPO and VOCLET Chemistry Model Set 1, JEXPO and VOCLET Chemistry Model Set 1, JEXPO and VOCLET Chemistry Model Set 1, JEXPO and VOCLET Chemistry Model Set 1, JEXPO and VOCLET Chemistry Model Set 1, JEXPO and VOCLET Chemistry Model Set 1, JEXPO and VOCLET Chemistry Model Set 1, JEXPO and VOCLET Chemistry Model Set 1, JEXPO and VOCLET Chemistry Model Set 1, JEXPO and VOCLET Chemistry Model Set 1, JEXPO and VOCLET Chemistry Model Set 1, JEXPO and VOCLET Chemistry Model Set 1, JEXPO and VOCLET Chemistry Model Set 1, JEXPO and VOCLET Chemistry Model Set 1, JEXPO and VOCLET Chemistry Model Set 1, JEXPO and VOCLET Chemistry Model Set 1, JEXPO and VOCLET Chemistry Model Set 1, JEXPO and VOCLET Chemistry Model Set 1, JEXPO and VOCLET Chemistry Model Set 1, JEXPO and VOCLET Chemistry Model Set 1, JEXPO and VOCLET Chemistry Model Set 1, JEXPO and VOCLET Chemistry Model Set 1, JEXPO and VOCLET Chemistry Model Set 1,